Infrared Laser Applications in Ophthalmology - Thermal Considerations
Alaa Mohammad Alghurab 1*, Nilofeer Ali Hammood Awadh 1, Ghaith Ali Muhammad 1, Ali H Alnasrawi 1,2, Ahmed Mohammed Khdhiar Amood 1, Hussein Safaa Abdulameer Alhassany 3, Maythum Ali Shallan 2 and Mohammed Abdullah Jassim 3
1Optics Techniques Department, College of Health and Medical Techniques, Al-Mustaqbal University, 51001 Babylon, Iraq
2College of Medicine, Al-Mustaqbal University, 51001 Babylon, Iraq
3Aesthetic and Laser Techniques Department, College of Health and Medical technique, Al-Mustaqbal University, Babylon 51001, Iraq
*Corresponding author
*Alaa Mohammad Alghurab, Optics Techniques Department, College of Health and Medical Techniques, Al-Mustaqbal University, 51001 Babylon, Iraq.
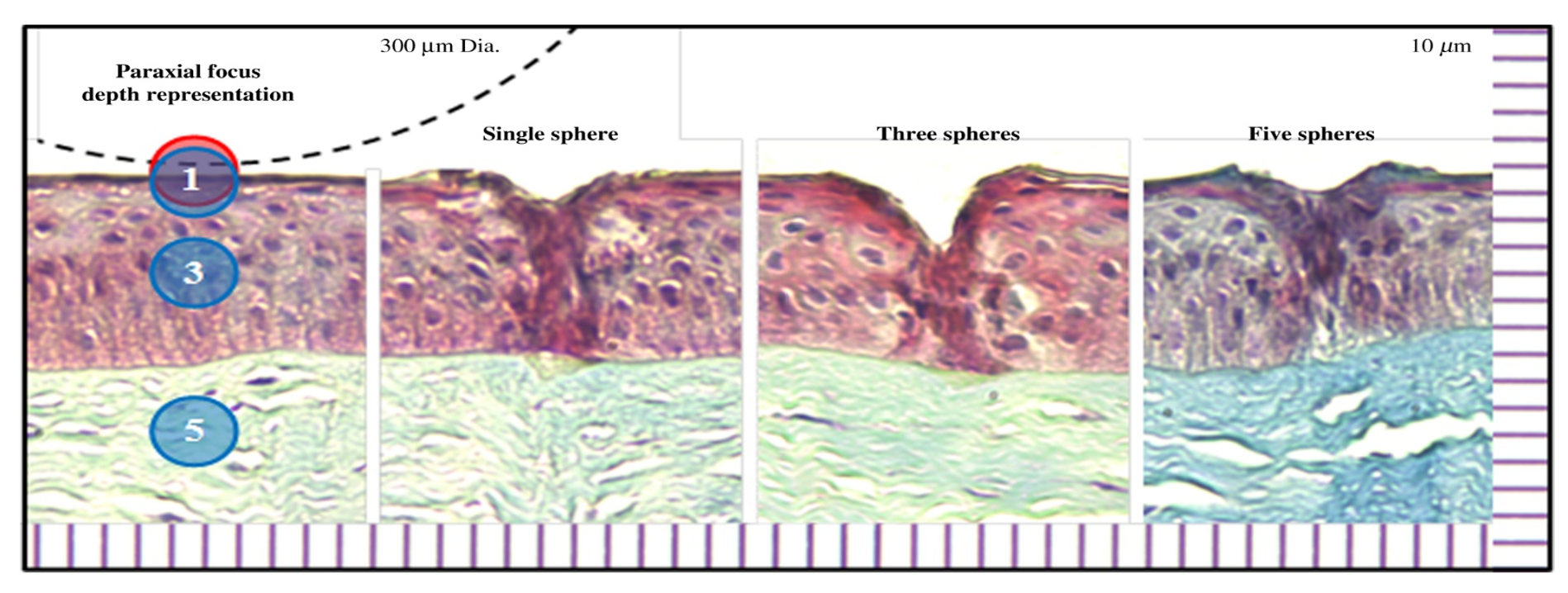
Figure 1: Trichrome histology of ablation craters created in the epithelium layer of porcine cornea using a detachable microsphere scalpel with integrated illumination. The dashed line illustrates the physical scalpel tip with 300-μm diameter for size comparison.
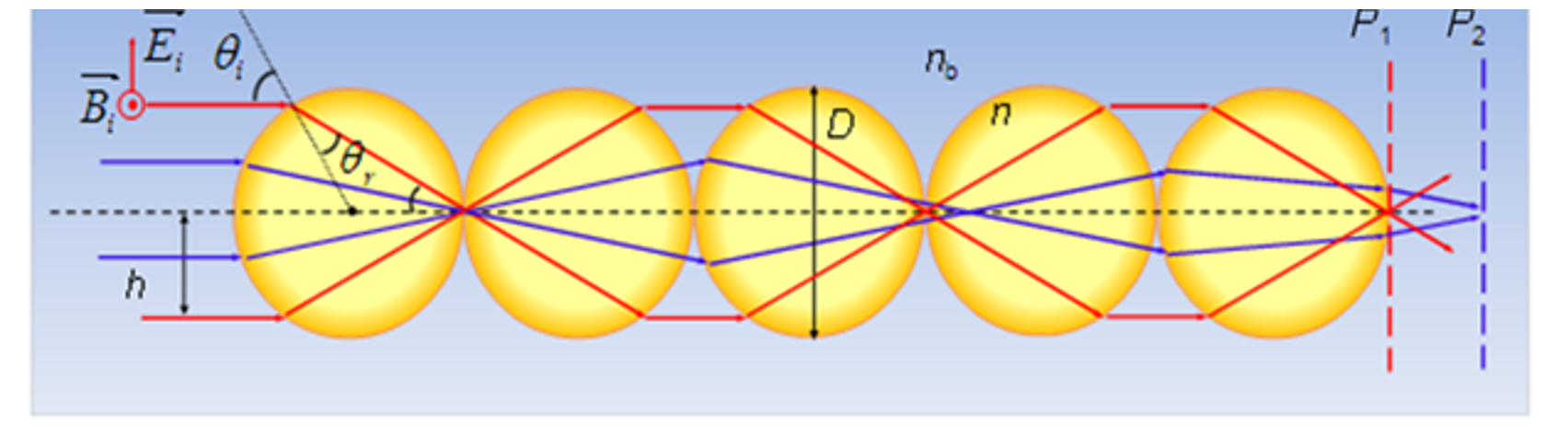
Figure 2: Different depth of focuses for different modes based on spherical aberrations in odd-numbered sphere chains.
- Gulias-Cañizo R, Rodríguez-Malagón ME, Botello-González L, Belden-Reyes V, Amparo F, Garza-Leon M (2023) Applications of infrared thermography in ophthalmology Life 13(3): 723.
- Zanellati G, Allegrini D, Auricchio F, Romano MR, Cattenone A, Alaimo G, Marconi S (2025) A review of optical and thermal eye tissue parameters for improved computational models in retinal laser therapy. Progress in Biomedical Engineering 7(1): 012008.
- Orsetti R (2023) Model-based planning and real-time monitoring for laser thermal therapy in ophthalmology. Doctoral Dissertation, Politecnico di Torino.
- Prasad A (2022) Laser techniques in ophthalmology: A guide to YAG and photothermal laser treatments in clinic. CRC Press.
- Prasad A (2022) Laser techniques in ophthalmology: A guide to YAG and photothermal laser treatments in clinic. CRC Press.
- Rahbar S, Abdelhalim I, Shokooh-Saremi M (2025) Modeling of the laser linear thermal effects on the anterior and posterior layers of the human eye with and without thermoregulation. Lasers in Medical Science 40(1): 1–15.
- Benson E, Fedele F (2022) Optical and laser techniques. In: Introduction to Medical Physics 385–421, CRC Press.
- Veysset D, Ling T, Zhuo Y, Pandiyan VP, Sabesan R, Palanker D (2022) Interferometric imaging of thermal expansion for temperature control in retinal laser therapy. Biomedical Optics Express 13(2): 728–743.
- Veysset D, Zhuo Y, Hattori J, Buckhory M, Palanker D (2022) Interferometric thermometry of ocular tissues for retinal laser therapy. Biomedical Optics Express 14(1): 37–53.
- Alghamdi NA, Youssef HM (2021) The thermal behavior analysis of a human eye subjected to laser radiation under the non-Fourier law of heat conduction. Journal of Heat Transfer 143(4):
- Pyo JY, Kim MC, Oh SJ, Mah KC, Jang JY (2025) Evaluation of optical and thermal properties of NIR-blocking ophthalmic lenses under controlled conditions. Sensors 25(11): 3556.
- Hameed H, Aqeel M, Rafid H, Sabah R, Hameed Z, Khalawi M, Shaker LM (2025) Transformative role of laser technology in ophthalmology and dermatology: A mini review of precision applications in modern medicine. AUIQ Complementary Biological System 2(2): 51–64.
- Lee KWA, Chan LKW, Lee AWK, Lee CH, Wan J, Yi KH (2024) Ocular complication in facial aesthetic laser and light treatments: A comprehensive review. Diagnostics 14(18): 2006.
- Abdulkareem AF, Hashim AQ (2024) Infrared medical thermography, medical applications, and its basic principles: A review. BIO Web of Conferences 97:
- Fried N, Seeber F, MacGregor T (2021) Therapeutic applications of lasers. Therapeutic Applications of Lasers.
- Chang DB, Luttrull JK (2020) Comparison of subthreshold 577 and 810 nm micropulse laser effects on heat-shock protein activation kinetics: Implications for treatment efficacy and safety. Translational Vision Science & Technology 9(5): 23.
- Wu W, Zhang H, Tao M, Li D, Wang G, Chen B, Zheng Y (2024) Experimental investigation on the transient vascular thermal response to laser irradiation by a high-speed infrared thermal camera. International Journal of Thermal Sciences 203:
- Omer H (2021) Radiobiological effects and medical applications of non-ionizing radiation. Saudi Journal of Biological Sciences 28(10): 5585–5592.
- Bodea F, Bungau SG, Bogdan MA, Vesa CM, Radu A, Tarce AG, Radu AF (2023) Micropulse laser therapy as an integral part of eye disease management. Medicina 59(8): 1388.
- Solyman OM, Hamdy O, Abdelkawi SA, Hassan AA (2022) Investigating the light emitting diode (LED) flashlight characteristics of a sample of smartphones for its safety in indirect retinal photography. Pan African Medical Journal 43(1).
- Valter K, Tedford SE, Eells JT, Tedford CE (2024) Photobiomodulation use in ophthalmology: An overview of translational research from bench to bedside. Frontiers in Ophthalmology 4: 1388602.
- Tetyczka C, Brisberger K, Reiser M, Zettl M, Jeitler R, Winter C, Roblegg E (2022) Itraconazole nanocrystals on hydrogel contact lenses via inkjet printing: Implications for ophthalmic drug delivery. ACS Applied Nano Materials 5(7): 9435–9446.
- Efron N (2025) Ocular thermography for optometric practice: A third reincarnation? Clinical and Experimental Optometry 108(5): 527–529.
- Elsayed MEA, Kozak I (2023) Basics of laser use in ophthalmology. In: Retina Lasers in Ophthalmology: Clinical Insights and Advancements 29–35, Springer International Publishing.
- Mei JH, Lin Z (2024) Subthreshold micropulse diode laser treatment in diabetic macular edema: Biological impact, therapeutic effects, and safety. International Ophthalmology 44(1): 3.
- Ye J, Wu Y, Pan J, Cai S, Cheng Y, Chu C, Su M (2024) ICG-based laser treatments for ophthalmic diseases: Toward their safe and rapid strategy. Luminescence 39(2): e4658.